All News
Hall Develops Device to Measure Gut Microbial Metabolism

The human intestines contain a complex and diverse community of microbes that break down food to produce metabolites. Some metabolites are beneficial to human health, such as vitamins, while others can be harmful and lead to obesity or gastrointestinal disorders. A major gap in the microbiome field is measuring and understanding these metabolites.
“There are currently few strategies to measure metabolites produced by the gut microbiome, and even fewer that work in real time,” says Brantley Hall, an assistant professor of cell biology and molecular genetics.
Now Hall has been awarded funding from the National Institutes of Health (NIH) to develop a wearable device that can do exactly that, potentially leading to therapeutic interventions for patients suffering from gastrointestinal disorders like Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease. IBS is one of the most common disorders seen by doctors, affecting one in 10 adults.
The $417K award will be spread over two years, supporting Hall’s work in the Center for Bioinformatics and Computational Biology (CBCB), a lab focused on research questions that arise from the genome revolution.
Hall, who also has an appointment in the University of Maryland Institute for Advanced Computer Studies, says that existing devices are much larger, and his goal is to design a smaller device that can perform the same functions if not better.
He adds that future developments could lead to personalized dietary interventions and improved communication between doctors and patients about their symptoms.
—Story by Maria Herd
Mihai Pop Reappointed as Director of UMIACS

Mihai Pop, a professor of computer science noted for his scientific research and advocacy for equality and inclusion in academia, has been reappointed as director of the University of Maryland Institute for Advanced Computer Studies (UMIACS) for a three-year term, effective July 1, 2022.
As director, Pop will continue to provide leadership to 76 UMIACS faculty members and almost 200 graduate students from eight departments across the UMD campus, as well as two-dozen administrative and technical staff that support the institute’s research, innovation and outreach.
Pop has served as director of UMIACS since November 2018, succeeding Amitabh Varshney, who is now dean of the university’s College of Computer, Mathematical, and Natural Sciences.
“Mihai has positioned UMIACS on a path of excellence,” Varshney said. “I look forward to continuing to work with him to advance the breadth and impact of computing research across our campus, the state of Maryland and beyond.”
With Pop as director, UMIACS has consistently brought in more than $25 million each year in research funding. The bulk of those external awards come from the numerous federal labs and agencies that dot the region.
Examples of projects funded under Pop’s tenure as director include the National Science Foundation’s support for computational epidemiology and fairness in artificial intelligence (AI); the National Institute of Standards and Technology’s funding for research in quantum information science and machine learning; and the Defense Advanced Research Projects Agency’s support of research into deepfake videos and AI system adaptation.
“I am honored and grateful to help guide an organization that has a substantive wealth of scientific knowledge and expertise to draw upon,” Pop said. “Much of our work is interdisciplinary, and all of it is focused on offering computational solutions that will have a positive societal impact.”
Moving forward, Pop plans to further strengthen UMIACS’ partnerships with federal agencies while simultaneously increasing activities with physicians and clinicians at the University of Maryland, Baltimore (UMB).
In 2019, Pop hosted a workshop between UMIACS faculty members and UMB researchers to advance new ideas involving artificial intelligence and health care. The initial results were promising, with several teams receiving awards to use AI in addressing aging, traumatic brain injury, mental illness and more.
Pop is also active in bringing more equality and diversity to the field of computer science and to academia in general. He instituted specialized workshops in UMIACS to support early-career faculty members and publicly called for greater recognition and support for this same group.
“The voice of a fresh Ph.D. assistant professor is often louder than that of the most experienced lecturer or research scientist on our campus,” Pop wrote in a 2018 op-ed article in The Faculty Voice. “It is high time for a change. Our campus cannot excel if we continue to ignore and undervalue the many faculty who shoulder the bulk of our teaching, and who represent a critical driving force in our research programs.”
Pop has continuously committed UMIACS resources in support of the Iribe Initiative for Inclusion and Diversity in Computing and other efforts that enhance participation in the discipline by people from diverse backgrounds. This includes sponsoring activities like Technica, the world’s largest hackathon for underrepresented genders; the Diversity in Computing Summit; and the Widening Natural Language Processing workshop.
“One of my top priorities as director of UMIACS is to increase the number of women in our institute,” Pop said. “The strength that comes from diversity—in gender, scientific background or academic titles—is the catalyst that propels new ideas and brings robust innovation.”
Pop’s own research interests cover several areas of bioinformatics, primarily related to the development of computational algorithms for analyzing biological data generated by high-throughput experimental techniques, such as sequencing technologies.
Part of Pop’s research focuses on the computational analysis of the microbial communities inhabiting our world and our bodies—a scientific field called metagenomics. His lab, part of UMIACS’ Center for Bioinformatics and Computational Biology, has developed a number of software tools that are now widely used in the field. He has also been an active participant in a number of large-scale, multinational projects, including the Human Microbiome Project and the GEMS study of diarrheal disease in children from the developing world.
In 2018, Pop made Clarivate Analytics’ list of Highly Cited Researchers, a compilation of influential names in science whose published work in their specialty areas has consistently been judged by their peers to be of particular use and significance. He was also included in the 2014 list.
In 2019, he was named a Fellow of the Association for Computing Machinery, an elite recognition of outstanding science and scholarship that is bestowed upon less than one percent of the organization’s 100,000-plus members. Earlier this year, he was named a Fellow of the International Society for Computational Biology in recognition of his work in developing algorithms for analyzing metagenomic data.
An active educator and mentor, Pop currently advises six graduate students and one high school student. He previously worked with 13 graduate students and six postdocs, and he spent three years as a faculty adviser to a group of UMD freshmen that analyzed the genome of the diamondback terrapin, the university’s mascot.
Pop earned his B.S. in computer science from University Politehnica of Bucharest in Romania in 1994 and his M.S.E. and Ph.D. in computer science from Johns Hopkins University in 1998 and 2000, respectively. He has been at UMD since 2005.
Researcher Awarded $1M From NASA to Develop Disease Forecasting Center and App

NASA has awarded $1 million to a renowned University of Maryland expert on cholera prevention and transmission to develop the first internet and app-based decision-making systems for infectious diseases, followed by a climate disease forecasting center.
The project led by Rita Colwell, a Distinguished University Professor in the University of Maryland Institute for Advanced Computer Studies, will be the application and culmination of her five decades of research on the bacterial infection of the small intestine, typically spread through contaminated water. Known as the 19th century’s pandemic, cholera has killed hundreds of millions of people and still takes 130,000 lives every year, mostly in underdeveloped countries with poor sanitation or areas hit by natural disasters.
Colwell discovered that the source of Vibrio cholerae bacteria is water abundant with plankton, and that it can linger there between epidemics by entering a state of dormancy. Her discoveries helped establish a new field—bacterial zoonosis—and changed scientists’ understanding of how diseases can be transmitted from aquatic organisms to humans.
She also developed state-of-the art techniques using satellite images to predict outbreaks, so that countries with limited budgets can mobilize health care workers and resources to stop the disease. Through remote sensing, computational biology and genomics, Colwell’s scientific advancements have saved thousands of lives and improved public health on a global scale.
Now her life’s work will become digestible in one app, and lay the framework for the first climate-related disease forecasting center, a collaboration that will include NASA, the United Nations, NOAA, the United Kingdom and the University of Florida. It will be located at the University of Maryland.
“This project is truly exciting as it will provide a means of predicting the risk of epidemics on a global basis. The impact may well prove to be enormous as it will enable pre-emptive action, notably to save lives,” she said. “Our newer models to be developed may be able to predict the intensity of given outbreaks, a very important factor for public health intervention.”
Antarpreet Jutla, an associate professor of environmental engineering at the University of Florida and Colwell’s co-principal investigator, said the $1 million award will support their long-term vision of predicting infectious diseases.
Leveraging his mathematical skills, her expertise in microbiology, and NASA’s satellite and weather data, Jutla and Colwell have over the past decade developed interactive maps for locations all over the world—the Chesapeake Bay, Zimbabwe, Mozambique, Senegal, Ethiopia, Bangladesh and Yemen—that can predict outbreaks with 90% accuracy.
Their sophisticated models are currently in use, with the latest prediction of an outbreak in war-torn Ukraine. Colwell also warns that “climate change is bringing cholera to new parts of the world, making this type of prediction system more important than ever before, especially because it provides an opportunity for early intervention.”
—Story by Maria Herd
Cummings Awarded $2.7M to Study Impacts of Aging on the Brain

Michael Cummings, a professor of biology with an appointment in the University of Maryland Institute for Advanced Computer Studies (UMIACS), is part of a team funded by the National Institutes of Health (NIH) to study how aging impacts the brain.
Working with medical experts at the University of Maryland, Baltimore (UMB), Cummings will continue his research on aging and traumatic brain injury in mice, which could eventually contribute toward treatments for age-related diseases like dementia and Alzheimer’s.
The $2.7M award will be spread across four years, supporting his team’s ongoing work that uses machine learning techniques to interpret “multiomics” data, where the data sets of different omics groups—genome, proteome, transcriptome, epigenome, and microbiome—are combined during analysis.
The collaboration was spurred by a state funding initiative called AI + Medicine for High Impact (AIM-HI), which joins faculty from both universities to tackle major issues that require cross-campus expertise in artificial intelligence and medicine. The initiative supports projects that have high potential for scientific breakthroughs and are likely to secure larger federal awards.
“The AIM-HI funding allowed us to show that machine learning could effectively be applied to multiomics data, with hundreds of thousands of variables quantifying the amounts of different proteins and lipids,” explains Cummings.
For their latest project, his team will develop software tools that can assess the brain’s changes in lipids and proteins, then characterize and test those tools.
Their results will be used to make inferences about biochemical pathways, which can help scientists identify potential interventions to mitigate the negative effects of aging on the brain.
Cummings notes that he met his UMB collaborators—Maureen Kane, an associate professor of pharmaceutical sciences, and Marta Lipinski, an associate professor of anesthesiology—at a meeting that was organized by UMIACS Director Mihai Pop intended to stimulate collaborative research proposals.
“Interdisciplinary research supported by new advances in computing will continue to open new doors of discovery,” says Pop. “This work by Michael and his collaborators in Baltimore is a perfect example of that.”
—Story by Maria Herd
CBCB Alums Named to TIME Magazine’s List of 100 Most Influential People

Two genomics experts who completed their doctoral training at the University of Maryland’s Center for Bioinformatics and Computational Biology (CBCB) were just named toTIME magazine’s annual list of 100 most influential people for their landmark work completing the sequencing of the human genome.
Michael Schatz, a Bloomberg Distinguished Professor of Computer Science and Biology at Johns Hopkins University (JHU), and Adam Phillippy, a bioinformatician at the National Human Genome Research Institute (NHGRI), were recognized for leading an international team in achieving the feat that scientists have been working toward for more than two decades.
Their team, the Telomere-to-Telomere (T2T) Consortium, used a mixture of new "long read" DNA sequencing technologies to map a gap-free sequence of the roughly three billion bases, or “letters,” in human DNA.
In making the announcement for TIME, Jennifer Doudna, a biochemist and winner of the 2020 Nobel Prize in Chemistry, explained that the team had uncovered the human genome’s “dark matter” that was missed by earlier sequencing.
“The complete human genome sequence is an invaluable resource that may provide new insights into the origin of diseases and how we can treat them,” Doudna wrote. “It also offers the most complete look yet at the genetic script underlying the very nature of who we are as human beings.”
The Human Genome Project announced it had finished decoding the basic chemical instructions for life in 2003, but skipped a significant section composed of highly repetitive sequences because they were mostly considered “junk DNA” at the time. Today, researchers know there’s more to it, which makes T2T’s work crucial.
Their findings were published earlier this year in the academic journal Science. Sergey Koren, another CBCB alum who completed his computer science Ph.D. in 2012, was a co-first author on the paper.
Phillippy and Schatz graduated with their Ph.D.s in computer science in 2010, working closely with their adviser Steven Salzberg. Now at JHU, Salzburg was CBCB’s founding director.
Schatz was co-advised by Mihai Pop, another genomics expert who is a professor of computer science and director of the University of Maryland Institute for Advanced Computer Studies.
“The success of the T2T project demonstrates the power of interdisciplinary science and represents the culmination of decades of advances in computational techniques for analyzing genomes, many of which were pioneered by Adam and Mike,” says Pop.
He adds that the pair fostered a collegial and collaborative environment in CBCB that still characterizes the center to this day, contributing to the success of many other outstanding scientists.
Looking forward, Schatz says he is excited about the possibilities of using their new genomic technology to improve many aspects of society, from agriculture to health care, and especially a better understanding of cancer.
Developing Algorithms to Predict the Spread of Infectious Diseases

The past 20 years have seen numerous epidemics break out across the globe: SARS, H1N1, Ebola, Zika, and most recently, COVID-19. These diseases have impacted the lives of billions, and lead to millions of deaths around the world. A multitude of factors can cause an infectious disease to develop and spread, including climate and poor sanitation, but even with knowledge of these factors it is difficult to predict when outbreaks will occur. Using previous research on the prediction of cholera outbreaks, however, researchers at the University of Maryland have developed algorithms that can predict the spread of infectious diseases from a local to global scale.
Early work on the prediction of infectious diseases began in the 1970s and 1980s, with the study of cholera in the Chesapeake Bay. Researchers then examined factors like water temperature and salinity as predictors of cholera outbreaks, and later studies integrated the use of satellite sensor data on a number of environmental and infrastructural measures as predictive factors.
Rita Colwell, Distinguished University Professor at the University of Maryland Institute for Advanced Computer Studies (UMIACS) and a pioneer in infectious disease outbreak prediction research—including the work above—sought to utilize and expand on prior satellite sensor models to forecast outbreaks of other diseases, including the risk of COVID-19 in the U.S.
The algorithms—developed using weighted averages from a public dataset—analyze several thresholds on weather, climate, and sociological, epidemiological, and environmental processes related to the abundance, survival, and emergence of new pathogens. The algorithms can also be applied to new pathways of pathogen emergence or transmission using common pathogen footprints. This tool can give public health officials a much-needed advantage to combat disease, and has the potential to help save millions of lives. Colwell expects that satellite sensors will become a major international public health tool to monitor communicable diseases.
The project was funded primarily by NASA, with funding also from the National Science Foundation, the British Aid Agency, and the United Nations. Colwell's collaborators include Anwar Huq, a research professor at UMD’s Department of Cell Biology and Molecular Genetics, and Antar Jutla from the College of Engineering, University of Florida.
Their invention, “Predictive Algorithms for Infectious Diseases,” was nominated for a UMD Invention of the Year award in the Information Sciences category. Winners were announced on May 3, 2022, at Innovate Maryland, a campus-wide celebration of innovation and partnerships at UMD.
This article was published by the University of Maryland Division of Research
Pop Named Fellow by International Society for Computational Biology

Mihai Pop, a professor of computer science and the director of the University of Maryland Institute for Advanced Computer Studies (UMIACS), is being recognized for his significant achievements in computational biology and bioinformatics.
The International Society for Computational Biology (ISCB) has selected Pop as a Fellow for 2022. He joins 10 other ISCB members who have distinguished themselves through outstanding contributions to the field.
ISCB selected Pop for his leadership in the development of algorithms for analyzing metagenomic data, particularly in the context of metagenome assembly and identification of structural variants in assembly graphs, and for his important contributions to large community projects.
Much of Pop’s research focuses on the analysis of microbial communities—a scientific field called metagenomics—which involves the application of bioinformatics tools to study the genetic material from environmental, uncultured microorganisms. Recent years have seen a “metagenomic revolution,” made possible by rapid advances in sequencing technologies, Pop says.
His lab, which is part of the Center for Bioinformatics and Computational Biology, has developed a number of software tools that are now widely used in the field.
They’re currently being funded by the National Institutes of Health to develop a suite of computational tools for reconstructing DNA segments.
The $2.6 million award is supporting Pop’s work building software and developing algorithms that can reconstruct nearly-complete microbial genomes from complex mixtures found in the human gut. He says that ultimately this will help scientists better understand the role of certain microbes in human health and disease.
Pop also leads efforts to assemble data at the Human Microbiome Project, which is aimed at surveying the complex microbial communities inhabiting the human body. His 122 publications have been cited(link is external) more than 38,000 times.
The 2022 Fellows will be recognized at the Intelligent Systems for Molecular Biology conference, held this year from July 10–14 in Madison, Wisconsin.
—Story by Maria Herd
Cummings Receives NVIDIA Academic Hardware Grant
Michael Cummings, a professor of biology with an appointment in the University of Maryland Institute for Advanced Computer Studies (UMIACS), recently received an NVIDIA Academic Hardware Grant to assist in the development of software that provides a rapid analysis of biological sequence data.
The grant is part of NVIDIA’s efforts to advance education and research by enabling groundbreaking, innovative, and unique academic research projects with world-class computing resources.
Cummings plans to use the NVIDIA hardware in the continued development of open-source software known as BEAGLE (Broad-platform Evolutionary Analysis General Likelihood Evaluator).
BEAGLE is an essential component in the software workflow of many scientists studying the evolutionary history of organisms, including the viruses that cause AIDS, influenza, Ebola, and now COVID-19. With the ability of high-throughput tools like BEAGLE to produce results quickly, researchers are helping public health agencies react to health threats, Cummings says.
He adds that the new hardware will allow for the development of new algorithms for larger data sets and more complex models.
“I am thankful to have current state-of-the-art hardware for students and other personnel to use in their research,” Cummings says. “As a mentor it is important that my students get experience with the best current hardware where it benefits their research and training. As a researcher, it is necessary to have the best current hardware to develop software and perform analyses at the limits of what is possible.”
Cummings, who is director of the Center for Bioinformatics and Computational Biology, was previously awarded a NVIDIA Global Impact Award to support his lab’s work on BEAGLE.
—Story by Melissa Brachfeld
Efficiently Processing Single-Cell and Single-Nucleus RNA-Sequencing Data
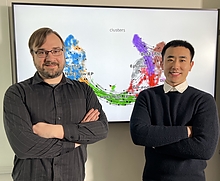
Caption: Associate Professor Rob Patro (left in photo) and Ph.D. student Dongze He (right) have released a toolkit for the efficient processing of single-cell and single-nucleus RNA sequencing data.
Rapid improvements in cell sequencing technologies in the last decade have provided clinicians and scientists with many valuable insights—from better treatment options for patients with heart disease and cancer to a much deeper understanding of how certain pathogens can affect plants and animals.
In particular, the exponential growth of high-throughput single-cell and single-nucleus RNA-sequencing technologies (collectively, single-cell transcriptomics technologies) have produced a wealth of new data. In fact, single-cell transcriptomic data constitutes the most ubiquitous components of single-cell multi-omics data, which was selected as the “2019 Technology of the Year” by the journal Nature Methods.
These technologies enable scientists to measure gene expression at the resolution of individual cells for tens or even hundreds of thousands of cells at a time. The measured gene expression can act as a crucial signal in understanding biological processes, disease progression, and even informing potential patient treatment options.
The result of this unprecedented resolution is that one can infer gene expression changes in all kinds of interesting biological contexts: How does gene expression differ between cells that respond to a drug versus those that are treatment resistant? How does gene expression differ among closely related cell types that happen to inhabit the same tissues within the body?
Single-cell sequencing has been a revolutionary tool in answering these kinds of questions.
But scientists must first “pre-process” this RNA-sequencing data—a crucial step that involves going from the raw sequencing data to a specific count of how abundant each gene is within each cell. And while there is popular commercial software available to accomplish this task, it is both time-consuming and memory intensive, as well as closed source.
Now, a multi-institutional team of researchers—including four with ties to the University of Maryland—has developed an accurate, computationally efficient, and lightweight toolkit for processing large amounts of raw single-cell and single-nucleus RNA sequencing data.
Their free suite of tools, called alevin-fry, is detailed in a paper published March 10 in Nature Methods.
“As the number and scale of single-cell, including single-nucleus, RNA-sequencing experiments grow, so do the costs associated with the processing of this data,” says Dongze He, a third-year doctoral student in computational biology at UMD and lead author on the paper. “Alevin-fry provides researchers an accurate, flexible and convenient way to process a multitude of types of single-cell data, simplifying and speeding up analysis and reducing computational costs of various single-cell related scientific activities.”
Instead of hours of processing time often requiring server-scale computers with large amounts of memory, the researchers say that their open-source toolset can process very large sets of single-cell data in only tens of minutes, using amounts of processing power and memory that is commonly available on commodity desktops and laptops, while retaining accurate results.
This exciting advancement is tied to a series of lightweight algorithms and efficient data structures, as well as a highly tuned implementation that can effectively make use of many processing threads at the same time. Applied in unison, these allow indexing a large amount of reference sequence—critical parts of the underlying genome—in small space, and quickly and accurately inferring the gene from which each sequencing read was generated.
The alevin-fry toolkit applies these lightweight approaches in a way that provides accurate results and should allow computational analysis to keep pace with the quickly-advancing biotechnology, says Rob Patro, an associate professor of computer science with an appointment in the University of Maryland Institute for Advanced Computer Studies.
“We think our software provides a compelling option for scientists working with single-cell and single-nucleus RNA-seq data, by enabling accurate and flexible gene quantification at a low computational cost,” Patro says. “As we receive feedback and input from other scientists using this method, we expect our software suite to grow in capability and comprehensiveness. Ultimately, we want this to be available to anyone looking for a seamless, efficient tool for advancing new discoveries using single-cell data.”
Other researchers working on the project are Mohsen Zakeri, who earned his doctorate in computer science from UMD in 2021 and is now a postdoctoral researcher at Johns Hopkins University; Hirak Sarkar, who earned his doctorate in computer science from UMD in 2020 and is now a postdoctoral researcher at Harvard Medical School; Charlotte Soneson, a research associate at the Friedrich Miescher Institute for Biomedical Research in Switzerland; and Avi Srivastava, a postdoctoral researcher at the New York Genome Center.
—Story by Melissa Brachfeld
Cummings and Resnik Receive MPower Seed Funding

Two faculty members in the University of Maryland Institute for Advanced Computer Studies (UMIACS) have received seed funding to advance interdisciplinary research focused on Parkinson’s disease, hearing disorders and antibiotic-resistant bacteria.
Michael Cummings (left in photo), a professor of biology, and Philip Resnik (right), a professor of linguistics, are active in three of the 17 projects recently chosen to split $3 million in seed funding from the University of Maryland Strategic Partnership: MPowering the State, known as MPower.
The MPower initiative was launched in 2012 to foster research, scholarship and innovation between the state’s top public research institutions, the University of Maryland, College Park, and the University of Maryland, Baltimore.
MPower’s competitive seed grant program—there were 52 proposals submitted this year—offers teams of faculty researchers grants of between $49,000 to $250,000. The money is intended to jumpstart high-impact research in areas that are of critical importance to the state of Maryland and the nation.
Cummings is principal investigator of a project that will use machine learning algorithms to help analyze mobility data from people suffering symptoms of Parkinson’s disease, a progressive nervous system disorder that affects nearly one million people in the United States.
He is joined on the project by Rainer von Coelln, M.D., an assistant professor of neurology at the University of Maryland School of Medicine.
The cross-institutional team will assess the severity of symptoms from 300 Parkinson’s disease patients in comparison to 50 control subjects who do not have Parkinson’s. All participants will wear sensors able to quantify and analyze their movements in their day-to-day activities or in specialized tasks they are asked to perform. Data to be analyzed includes symptoms like slowness, stiffness and shaking, mental health issues like anxiety/depression, and so-called autonomic symptoms like bladder dysfunction and dizziness.
The severity of these symptoms—and their rate of progression—often varies between patients. The captured sensor data analyzed by sophisticated algorithms could help clinicians quickly identify which Parkinson’s patients need more aggressive treatment protocols to help prevent their rapid deterioration.
Cummings is also involved in a second MPower-funded project being led by Matthew Goupell, a professor of hearing and speech sciences at UMD, and Ronna Hertzano, M.D., an otolaryngologist surgeon-scientist at the University of Maryland School of Medicine.
In this project, the researchers are developing innovative tools to effectively query and analyze hearing loss data for research purposes.
They plan to adapt a cloud-based tool developed at University of Maryland to support audiological clinical research data visualization and analysis. Ultimately, this could result in an advanced and intuitive clinical informatics tool that would assist in the implementation of multi-site clinical studies for hearing disorders.
Resnik is collaborating on an MPower seed grant with Katherine E. Goodman, an assistant professor of epidemiology and public health at the University of Maryland School of Medicine.
Their joint UMD/UMB team is interested in addressing the spread of antibiotic-resistant bacteria that can pose a grave challenge in U.S. hospitals.
One concern, the researchers say, is that colonized (i.e., “silent” carrier) patients often go undetected, transmitting antibiotic-resistant bacteria to other patients. Moreover, the colonized patients themselves are at a significantly higher risk of developing antibiotic-resistant infections, where mortality rates can exceed 50%.
But wide-scale screening for antibiotic-resistant bacterial colonization remains impractical for most U.S. hospitals. As a result, hospitals can miss critical opportunities to identify colonized patients early, when it is still possible to prevent what are often devastating patient outcomes.
The MPower-funded team is pursuing an innovative strategy: using state-of-the-art natural language processing and machine learning techniques to analyze the language in electronic health records, automatically detecting pre-admission exposures that might be found in a patient’s clinical notes.
Those notes—showing someone arriving at the hospital from a nursing home, for example—can often yield important information on strong risk factors for carrying antibiotic-resistant bacteria.
The research team aims to lay foundations for automated technology that will detect these high-risk patients in a much more targeted and cost-effective way than is currently available.
This is the second round of MPower seed funding for Resnik, who in 2016 was awarded a grant with UMB Professor of Psychiatry Deanna Kelly to develop computational models that help identify symptomatic changes in people suffering from schizophrenia or depression. The 2016 MPower grant was followed by an $842,000 National Science Foundation award in 2021.
